London
Roots Analysis has announced the addition of the “At home testing Kits Market , 2022–2035” report to its list of offerings.
Owing to several advantages offered by at-home self-testing kits for diagnostics and screening purposes, industry stakeholders have undertaken numerous RD initiatives focused on exploiting the use of such kits to provide healthcare at a fraction of a cost to the general public when compared to traditional clinics. Associated cost saving and privacy are main drivers of the growth of this domain over the coming decade. The ongoing innovations in this market are anticipated to bring a positive revolution in the healthcare domain. Given the continuous efforts being undertaken by industry players, the at-home self testing kits market is poised to witness healthy growth over the next decade.
Key Market Insights
Presently, more than 51% of the total at-home self-testing kit developers are based in North America
This segment of the industry is dominated by the presence of companies that were established pre-2000 (37%), followed by firms established during 2000-2010 (32%). In addition, most of the players engaged in this domain are very small and small firms (less than 100 employees), representing close to 60% of the total kit developers.
Over 500 at-home self testing kits are currently available in the market
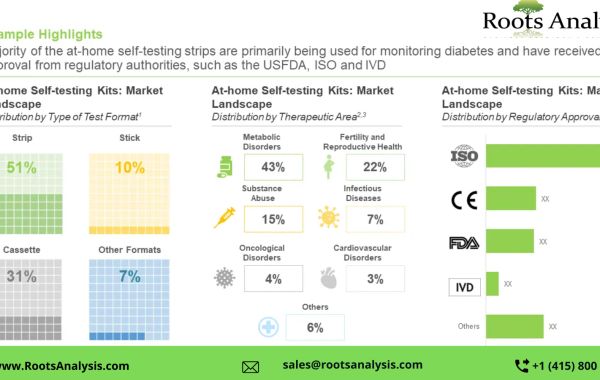
It is worth noting that most of the at home testing kits are currently being used for monitoring disorders (43%), followed by those employed for detection of conditions related to fertility and reproductive health (22%). Further, most of the kits are being developed to follow invasive route for the procurement of analyte (56%), followed by those being manufactured to utilize non-invasive methods for procuring analyte (43%).
More than 1,130 grants have been awarded to support the ongoing research efforts in this domain
Several non-profit organizations have extended financial support to aid the research related to at-home self-testing kits. It is worth mentioning that NIAID emerged as the most prominent non-profit organization, having awarded over 23% of grants for a period of 1-5 years, followed by NHLBI (12%).
The demand for at-home self-testing kits is projected to grow at a rapid pace, in the foreseen future
It is worth highlighting that the demand for HIV screening kits is expected to grow at a rate of 13%. Further, the highest demand for such products is expected to be driven by the Asia-Pacific region in 2035 (56%).
The market is poised to be well distributed across different types of biofluids analyzed, till 2035
The current market is driven by kits developed for metabolic disorders (64%); this trend is unlikely to change in the foreseen future as well. Further, based on type of test format, strip-based tests are likely to have the maximum revenue generation potential (50%), by 2035.
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/self-testing-kits-market/request-sample.html
Key Questions Answered
- Who are the leading players engaged in the manufacturing of at-home self-testing kits?
- What is the most common diagnostic assays offered by at-home self-testing kit manufacturers?
- What are the key regulatory frameworks for at-home self-testing kits across different regions?
- What are the key factors influencing the price of at-home self-testing kits?
- What is the likely valuation / net worth of companies engaged in this domain?
- Which companies / institutes have received grants for research and development of at-home self-testing kits?
- What is the current, global demand for at-home self-test kits?
- How is the current and future market opportunity likely to be distributed across key market segments?
- What is the current market scenario (in terms of existing competition and growth opportunities) across emerging and established market segments?
The financial opportunity within the at-home self-testing kits market has been analyzed across the following segments:
- Type of Test Format
- Strip Format
- Stick Format
- Cassette Format
- Others
- Type of Biofluid Analyzed
- Blood
- Urine
- Stool
- Target Therapeutic Area
- Metabolic Disorders
- Fertility and Reproductive Health
- Infectious Diseases
- Oncological Disorders
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Africa
- South America
The report includes profiles of key players (listed below); each profile features an overview of the company, details related to its at-home self-testing kits focused portfolio, recent developments, and an informed future outlook.
- ACON Laboratories
- AdvaCare Pharma USA
- Apex Biotechnology
- i-SENS
- Oak Tree Health
- Tai Doc Technology
- VivaChek Laboratories
For additional details, please visit
https://www.rootsanalysis.com/reports/self-testing-kits-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Viral Vaccine Cell Culture Media Market: Industry Trends and Global Forecasts, 2022-2035
- Next Generation Sequencing (NGS) Kits Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091