As an expert in providing solutions to assist virology and microbiology research, Creative Diagnostics is proud to announce the launch of its comprehensive portfolio of ELISA Based Assays for vaccine development and bioprocess applications.
Vaccine development involves a variety of assays to support vaccine research and development, from early preclinical studies to final production, quality control and lot release. ELISA-based assays are widely used to measure vaccine titers, carrier titers, purity, affinity and potency, as well as immunogenic responses in animals and humans.
ELISA-based immunoassays are the gold standard for various aspects of vaccine bioanalysis, including quantification of antigenic epitopes, detection of contaminants, and determination of vaccine efficacy (e.g., vaccine titer and immunogenicity). They are crucial for vaccine bioanalysis, but limitations exist, such as high reagent and sample consumption, diverse sample types, and time-consuming procedures. To address these issues, researchers are exploring improved immunoassays to minimize the use of valuable reagents (e.g., vaccine candidate molecules) and small animal samples, handle different sample types such as serum, plasma, and preparations containing aluminum particles and emulsions, and reduce hands-on time to shorten assay development time.
Creative Diagnostics is committed to providing innovative and reliable solutions to advance vaccine development and bioprocess efficiency. With its expertise in ELISA-based assays, the company empowers researchers and manufacturers to effectively measure vaccine titer, vector titer, purity, potency, and immunogenic response, ultimately contributing to the development of safe and effective vaccines.
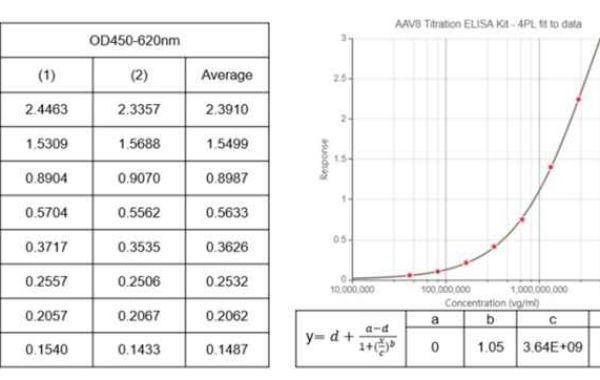
For example, Creative Diagnostics can help researchers determine viral vector particle concentrations (titers). Viral vector vaccines are live viruses genetically engineered to express one or more heterologous antigens. Determination of vector particle concentration is an important step in the development and production of such vaccines. Creative Diagnostics now offers high quality ELISA kits for the determination of titers of a range of AAV serotypes or lentiviruses (p24).
In addition, Creative Diagnostics can assist in the detection of antigen-specific antibodies in serum and in antigen quantification and comparison of vaccine lots for release testing. By providing expertise and comprehensive solutions, Creative Diagnostics enables researchers and manufacturers to advance vaccine development and bioprocess efficiency.
Creative Diagnostics also offers a comprehensive range of high-quality ELISA kits for the detection of viral antigens and antibodies. With extensive experience in custom assay development and validation, the Creative Diagnostics team of experts ensures customized solutions to support customer needs. Utilizing extensive reference control and standard materials, Creative Diagnostics delivers reliable qualitative and quantitative testing. Additionally, the company’s holistic study design considers pretesting requirements, sampling modes, detection methods, and assay sensitivities, resulting in effective viral ELISA-based assays.
By offering a comprehensive suite of ELISA-based solutions and unparalleled scientific expertise, Creative Diagnostics empowers researchers and developers to streamline their vaccine development and bioprocess workflows. For more information on the ELISA Based Assays, please visit https://antiviral.creative-diagnostics.com/elisa-based-assays.html.
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.