Abbe Refractometer Measurement Experiments Analysis
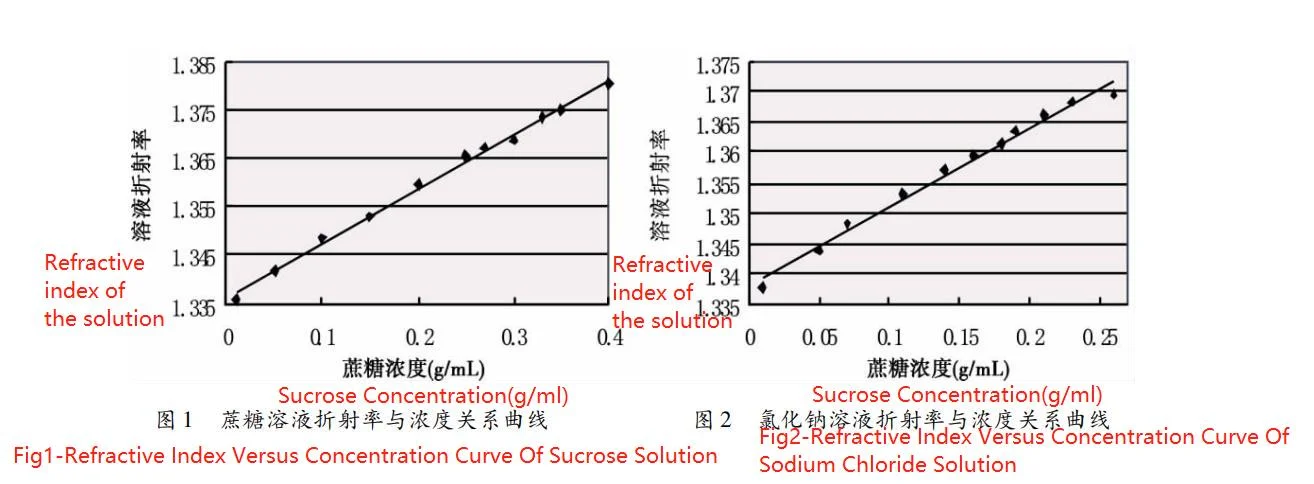
The refractive indices of sucrose solution and sodium chloride solution at room temperature (20 ℃) are shown in Table 1. From Table 1, it can be seen that the refractive indices of both solutions increase with increasing concentration. For this reason, the data number 1, 3, 5, 7, 9, 11, 12, 13, 14, 15, and 17 were fitted by linear least squares regression, and the rest of the data were used as the validation set to test the prediction accuracy of the model. The fitting results are shown in Fig. 1 and Fig. 2, respectively.
n = 0. 1117c + 1.3363——Formula(1)
The correlation coefficient is 0. 9978. The linear relationship between the refractive index n of sodium chloride solution and its concentration c is given by:
n = 0.1312c + 1.3379——Formula(2)
The correlation coefficient is 0.9937. The fitting results show that there is a significant linear relationship between the refractive indices of both solutions and their concentrations. Moreover, when the concentration is zero, the refractive index of the solution is very close to that of distilled water, so the experiment is successful. Meanwhile, Table 1 shows that the refractive indices of the solutions are comparable to those of pure water at very low concentrations. Therefore, the detection limit of the method can be roughly determined at the level of 0.010 g/mL. The overall root-mean-square error of sucrose concentration prediction was 0. 013 g/mL; the overall root-mean-square error of sodium chloride concentration prediction was 0. 007 g/mL. The prediction accuracy was found to be in accordance with the requirements.
Table1 Refractive Index Of Single Component Solution ( Concentration Unit: g /Ml, Room Temperature: 20 ℃ )
Number | Sucrose Concentration | Refractive Index Average | Sodium Chloride Concentration | Refractive Index Average |
1 | 0.010 | 1.336 0 | 0.010 | 1.3376 |
2 | 0.030 | 1.3387 | 0.030 | 1.3404 |
3 | 0.050 | 1.3417 | 0.050 | 1.3437 |
4 | 0.070 | 1.3435 | 0.060 | 1.3450 |
5 | 0.100 | 1.3487 | 0.070 | 1.3482 |
6 | 0.130 | 1.3520 | 0.090 | 1.3501 |
7 | 0.150 | 1.3530 | 0.110 | 1.3532 |
8 | 0.170 | 1.3574 | 0.130 | 1.3558 |
9 | 0.200 | 1.3597 | 0.140 | 1.3570 |
10 | 0.230 | 1.3634 | 0.150 | 1.3587 |
11 | 0.250 | 1.3654 | 0.160 | 1.3594 |
12 | 0.270 | 1.3673 | 0.180 | 1.3614 |
13 | 0.300 | 1.3686 | 0.190 | 1.3636 |
14 | 0.330 | 1.3734 | 0.210 | 1.3662 |
15 | 0.350 | 1.3748 | 0.230 | 1.3684 |
16 | 0.370 | 1.3579 | 0.240 | 1.3689 |
17 | 0.400 | 1.3805 | 0.260 | 1.6395 |
The results of refractive index measurements of the two-component solutions are shown in Table 2. The numbers 1, 3, 5, 7, 9, 11, 12, 13, 14, 15, and 17 were selected for the binary linear regression of the refractive index of the solution on the concentration of the components, and the remaining numbers were used as the validation set. The binary linear regression was carried out in Excel software, and the regression equations of the refractive index of solution n with sucrose concentration c1 and sodium chloride concentration c2 were obtained as:
n = 0.0759c1 + 0.0804c2 + 1.3518——Formula(3)
Table2 Refractive Index Of Two-Component Solution ( Concentration Unit: g /Ml, Room Temperature: 20 ℃ )
Number | Sucrose Concentration | Sodium Chloride Concentration | Refractive Index Average |
1 | 0.400 | 0.010 | 1.3826 |
2 | 0.370 | 0.030 | 1.3821 |
3 | 0.350 | 0.050 | 1.3817 |
4 | 0.330 | 0.060 | 1.3816 |
5 | 0.300 | 0.070 | 1.3812 |
6 | 0.270 | 0.090 | 1.3799 |
7 | 0.250 | 0.110 | 1.3800 |
8 | 0.230 | 0.130 | 1.3806 |
9 | 0.200 | 0.140 | 1.3786 |
10 | 0.170 | 0.150 | 1.3767 |
11 | 0.150 | 0.160 | 1.3753 |
12 | 0.130 | 0.180 | 1.3766 |
13 | 0.100 | 0.190 | 1.3749 |
14 | 0.070 | 0.210 | 1.3737 |
15 | 0.050 | 0.230 | 1.3741 |
16 | 0.030 | 0.240 | 1.3731 |
17 | 0.010 | 0.260 | 1.3733 |
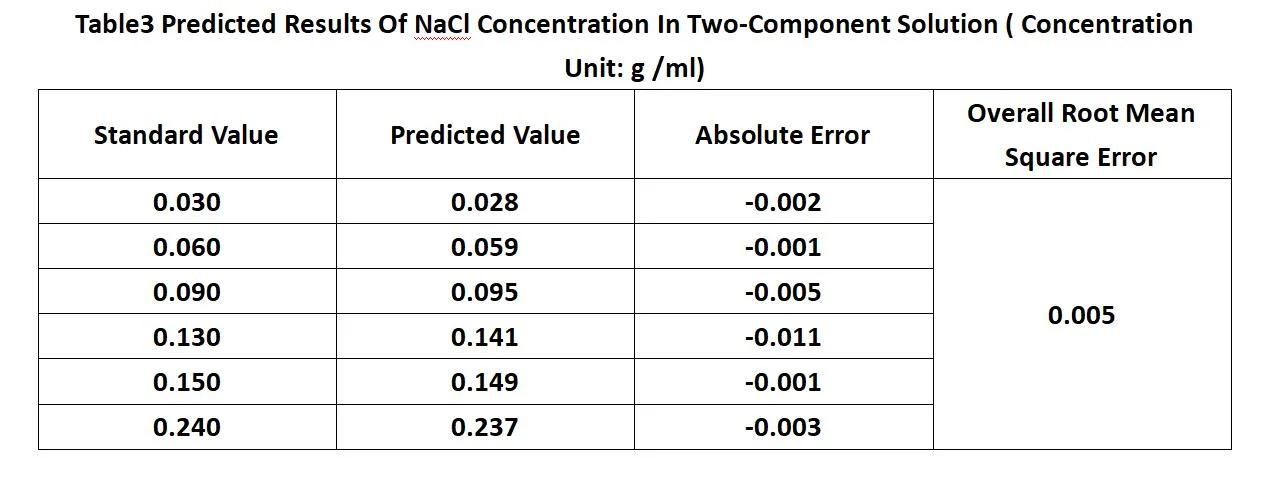
The complex correlation coefficient is 0. 988 2, the coefficient of determination is 0. 9766, the corrected coefficient of determination is 0. 970 8, and the estimated standard error is 0. 000 6. The statistical F value is 167. 204, and the significance level is 2. 98E-07, which means that the regression equation is highly significant and linear. For a two-component solution, if the concentration of one of the components is known, the regression equation can be used to predict the concentration of the other component by measuring the refractive index of the solution. For the selected validation set, assuming that the sucrose concentration in the solution is known (e.g., measured by the spin method), the prediction results from the regression equation are shown in Table 3. The predicted values are very close to the standard values, and the overall root means an error of the prediction is 0.005 g/ml, indicating that the model has a high prediction accuracy.