As an expert in providing solutions to assist virology and microbiology research, Creative Diagnostics has recently launched a series of Antimicrobial Synergy Testing Services to the research community, including Standard Bacteria Culturing, Antimicrobial Synergy Testing, and Statistics Analysis of experimental results.
Creative Diagnostics works with scientists in industry and academia to support the early stages of antimicrobial, vaccine and diagnostic discovery and to advance research in bacterial infections. The company can provide assays for some of the major bacterial families, help customers select the appropriate assay based on compound and mechanism of action, and develop custom assays based on the customer's specific process.
The antimicrobial synergy testing/checkerboard test is an experimental method used to evaluate the interaction of two antimicrobial test compounds. In this test, the MIC and MBC values of the test compounds are used individually and combined with the MIC and MBC values of each bacterial strain being evaluated to calculate accumulation.
Synergy measurements by checkerboard analysis can be used to determine the change in antimicrobial potency of an antibiotic combination relative to its individual activity. Comparative results are then calculated using the Fractional Inhibitory Concentration (FIC) index value, which represents the greatest change in antibiotic combinations compared to the MIC of a single antibiotic.
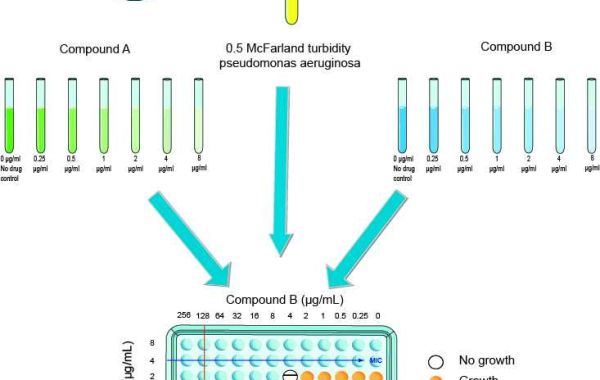
Creative Diagnostics offers microdilution checkerboard assays to assess the interaction of two or more antimicrobial agents against specific pathogens. The first step is to prepare stock solutions of each drug and two consecutive dilutions, leading to at least double the MIC. Then a total of 50μl of Mueller-Hinton broth is dispensed into each well of the microdilution plate. The first antibiotic in the combination is diluted sequentially along the ordinate, while the second drug is diluted along the abscissa. An inoculum equivalent to 0.5 McFarland turbidity standard is prepared from each Pseudomonas aeruginosa isolate in Mueller-Hinton broth.
100μl of 5 x 105 CFU/ml bacterial inoculum is then inoculated in each microtiter well, and the plate is incubated at 35°C for 48 hours under aerobic conditions. The resulting checkerboard grid contained each combination of the two antibiotics and diagonally the tubes with the highest concentration of each antibiotic. Finally, to quantify the interaction between the tested antibiotics (FIC index), the following formula was calculated: A /MIC A + B/MIC B = FIC A + FIC B = FIC Index.
By combining infectious disease and analytical expertise, Creative Diagnostics provides clients with the most robust portfolio of antiviral and antibacterial in vitro testing services. As the demand for novel antiviral and antimicrobial compounds to treat infectious diseases continues to grow, Creative Diagnostics can help clients test compounds in vitro to determine their potential efficacy in in vivo models.
Creative Diagnostics provides high-efficiency, high-quality, competitive-priced, 24/7 online antimicrobial synergy detection services with timely result feedback. For more information, please visit https://antiviral.creative-diagnostics.com/antimicrobial-synergy-testing-checkerboard-assay.html.
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.