CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, is pleased to announce a suite of comprehensive mRNA-LNP Vaccine Laboratory Process Development Assays. This latest addition to CD Bioparticles' extensive service portfolio is specifically designed for the rapid and efficient development of mRNA-LNP vaccines.
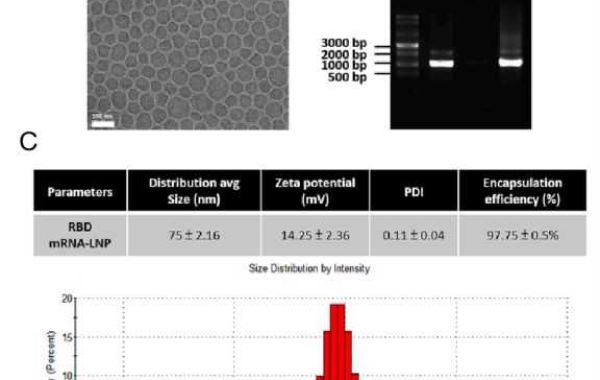
The mRNA molecule is well known to the public through COVID-19 and the mRNA vaccine made from it is characterized by rapid blocking of pathogen mutation response, simple production process and scale-up. Detection and validation methods are the basis for quality control of mRNA LNP vaccines. Therefore, RD companies should establish detection methods for the characteristics of mRNA, liposomes and formulations, as well as related impurities and other key quality attributes of mRNA vaccines to ensure the safety and efficacy of mRNA-LNP vaccines.
CD Bioparticles now offers a comprehensive portfolio of laboratory assays designed to support every stage of mRNA-LNP vaccine development. These assays provide researchers with the tools they need to optimize their vaccine formulations and ensure their safety and efficacy, accelerating the path to clinical trials and commercialization. In addition, CD Bioparticles’ team of scientific experts is available to work with researchers to develop custom assays to meet their specific needs.
CD Bioparticles mRNA-LNP vaccine assay portfolio encompasses a variety of methodologies, including Cellular Uptake Studies,mRNA Transfection Efficiency Study, In Vivo Biodistribution, Histopathological Studies, Biochemical Indicators Testing, and Detection of Antigen-Specific T Cells. By leveraging CD Bioparticles’ expertise in analysis and characterization, researchers can gain deeper insights into the properties and performance of their vaccines, enabling them to make informed decisions during the development process.
For example, in the mRNA Transfection Efficiency Study, DC2.4 cells are selected as the model cells for the infection experiment. After infecting the cells with GFP-mRNA-LNP, the intracellular GFP signal will be observed by fluorescence microscopy. CD Bioparticles can assist customers with immunostaining studies that not only examine the efficiency of mRNA transmission, but also help to identify the specific cell types that are transfected into a specific type of organ.
CD Bioparticles also offers In Vivo Biodistribution services. To visualize the distribution of LNP in an organism, the professionals use fluorescent dyes (e.g., DiD and DiO) and luciferase for labeling. Typically, luciferase mRNA (Luc-mRNA) is used as a model mRNA to follow the distribution of the expressed protein in vivo. After intravenous injection of Luc-mRNA-LNP, heart, liver, spleen, lung, kidney and lymph node tissues are isolated. The tissues will be visualized using the IVIS Spectral Live Image System. This will facilitate visualization studies of organ distribution and duration of protein activity production.
CD Bioparticles' new mRNA-LNP assays are available to researchers and vaccine developers worldwide. The company is committed to providing cutting-edge technologies and services that support the advancement of biotechnology and improve human health. For more information about the services, please visit https://www.cd-bioparticles.net/services/bioparticles-analysiscand-characterization/mrna-lnp-vaccine-laboratory-process-development-assay.
About CD Bioparticles
CD Bioparticles is an established drug delivery company that provides customized solutions for developing and manufacturing novel biocompatible drug delivery systems. It specializes in various formulation and drug delivery technologies, from conventional liposomes and PEGylated liposomes to polymer microspheres and nanoparticles for drug delivery. The company also provides contract research services for drug delivery formulation, formulation feasibility study, process development and scale-up, as well as analytical and non-clinical research services.